IACFS International Conference Summary
11th International IACFS/ME Conference
Parc 55 Wyndham Union Square Hotel
San Francisco CA
March 20-23, 2014
The theme for this biennial IACFS conference was “Translating Science into Clinical Care,” which implies that most papers would address treatments and management schemes aimed at improving the care of persons with CFS/ME and FM. Now I do not want to sound negative… this meeting is always highly anticipated and much appreciated because every 2-3 years it brings together notable researchers and clinicians to share their interim work. But disappointingly there were few presentations on treatment, per se, and really no new ideas. There were no new medications, no new breakthroughs in management that clinicians like myself could take home to my eager patients. We didn’t learn about any miracle antivirals or new immune therapies, and there was little mention at all of hopefuls like rituximab, for example, and only one poster pointed out that a subset of patients respond to the investigational drug rintatolimod/ Ampligen with a significant improvement in exercise tolerance compared to controls.
That said, let me summarize a few of the presentations that I believe were most meaningful. I will add my own comments periodically, hoping that this will help your understanding:
Dr. Ian Lipkin kicked off the professional portion of this meeting with the encouraging news that his esteemed team of researchers at Columbia University will be actively seeking a cause for CFS/ME. Entitled “Small Game Hunting,” Lipkin detailed how he and his teams discovered the West Nile Encephalitis Virus and 400 other infectious agents. In addition, they have been called in as “medical sleuths” to solve other baffling illnesses, such as Pork Packers Encephalitis, which was found due to toxic myelin particles in the air at meatpacking facilities, and not due to an infectious agent at all! His research team utilizes virology, immunology, metabolomics, genomics, studies of gut bacteria, and even atmospherics to solve medical mysteries. They have unraveled how excess sugar affects the gastrointestinal biome of children with autism; that high fiber diets reduce the risk of colon cancer by increasing gut butyrate; and that Kawasaki’s vasculitis is caused by candida species that drops from the troposphere onto susceptible individuals. So it is very encouraging to have this man and his world-renowned team on our side, looking for the cause of this dread disorder — CFS/ME.
Therapy
The first presentations of the conference focused most closely on therapeutics. Dr. Jose Montoya (Stanford) briefly reviewed current antiviral therapies for herpes viruses, specifically acyclovir/Zovirax*, valcyclovir/Valtrex*, famcyclovir/FamVir*, gancyclovir, valgancyclovir /Valcyte*, foscarnet, and cidofovir. The oral agents in this series ( marked *) are currently used when herpes virus titers are exceptionally high. He pointed out that the proper doses are unknown, treatment may initially worsen symptoms, and treatment had to be continued for 6-12 months in most cases. I would add that the response to these therapies has been modest at best.
Dr. Nancy Klimas (Nova Southeastern University) then addressed specific targeting of immune abnormalities. The cytokine, Tumor Necrosis Factor (or TNF-alpha), is frequently elevated in persons with CFS (PWCs), and can be suppressed by biological agents such as infliximab/Remicade, edalibumab/Humira, etanercept/Enbrel, and others. These are very expensive, however, and not approved for use in CFS/ME. In persons with concurrent CFS/ME and rheumatoid arthritis or psoriasis (for which they are indicated), such treatment could be beneficial. Other cytokine inhibitors have been developed but systemic administration of these drugs frequently engenders multiple severe side effects.
Persons who have concurrent CFS/ME and recurrent respiratory infections due to antibody deficiencies may benefit from administration of gamma globulin. Again this is very expensive and partially covered by insurance when specific conditions are met.
Klimas pointed out that low dose naltrexone and inosine/Isoprinosine are currently available immune modulators, and studies of rintatolimod/Ampligen, rituximab/Rituxan, and Interferon-alpha and -gamma are ongoing.
A poster presentation from Klimas’s Institute for Neuro-Immune Medicine in Davie FL reported that EBV Early Antigen positivity (a sign of Epstein Barr Virus reactivation) occurred equally in PWCs and healthy controls, surprisingly, but the fatigue level in PWCs was much higher in EBV-EA positive individuals, which might be a good indication for anti-viral treatment.
The use of midodrine/ProAmatine in orthostatic intolerance and Neurally Mediated Hypotension was studied by Nicole Baldwin, a student visiting Dr. Bateman’s clinic in Salt Lake City. PWCs who used midodrine, an alpha agonist that increases blood pressure, were able to stand longer (increased Hours of Vertical Activity) and had fewer symptoms (such as headache, fatigue, and cognitive impairment) than those who elected not to use it.
For those interested in Eastern techniques, Dr. Tian-Fang Wang (Beijing University of Chinese Medicine) described the benefits of Baduanjin, a form of tai chi. Participants who exercised twice daily for 6 weeks had improved mental and physical fatigue, and better sleep than a control group. Likewise, Takakazu Oka presented a controlled trial of isometric yoga in addition to conventional treatment and cognitive therapy. Participants reported less fatigue and increased vitality. Lab studies showed an increase in DHEA and reduced prolactin in participants, but no significant changes in acyl-carnitine, BDNF, TGF-b, IL6, TNF-a, or MSH. [Ed. Note: many of our patients report that such activities are easy to perform, infrequently trigger flares, and do provide great benefit.]
The Chronic Fatigue Initiative (CFI) is a consortium of several clinics supported by the Hutchins Family Foundation. One of many studies by this group was “What Treatments Alter the Course of CFS.” 1430 PWCs from four geographically distinct areas were interviewed by trained individuals as to what treatments helped the most. The most effective treatments were self-help strategies (65%) and traditional medicine (53%). Effective self help strategies included rest (37%), diet modifications (21%), and low level exercise (19%). Beneficial traditional therapies were symptomatic medications (38%) and vitamins (16%). Only a small proportion reported benefit from complimentary and alternative medicine such as herbal remedies (7%) and acupuncture/reflexology/massage (7%). [Ed. Note: I hate to say it, but “I told you so!” Although many would expect to hear that antivirals, immune modulators, or alternative therapies would top the list, the Stepwise Approach that we have espoused for years continues to be the best and most successful approach.]
Dr. Katherine Rowe (Royal Children’s Hospital, Melbourne AU) examined what young people find most helpful. They reported back that apportioning their limited energy across four domains (socialization, education, physical activity, and out-of-home activities) was most important, and of those four domains socialization was most crucial. Helpful interventions included massage for muscle pain, good dietary advice, psychological support, and support groups or chat rooms. It was most important that friends and family “believe me,” and that they had ongoing support at home and in navigating the educational system.
Unhelpful were “not being believed,” delays in diagnosis, being forced to exercise, inflexible teachers and school administrators, promises of a cure, being told “it’s all in your mind,” and doctor shopping. The best predictor of a good outcome in adolescents was continuing education, as many adolescents worry considerably about being able to support themselves later on.
Immunology
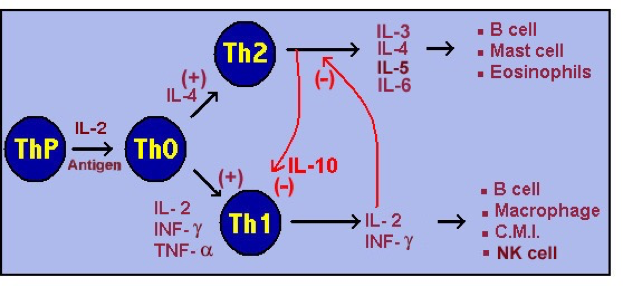
As in the past, there was a great deal of discussion about immunology and cytokine patterns in CFS. By way of introduction, let me explain that the immune response is currently divided into at least three major “types” or “responses”: Th1, Th2, and Th17. “Th” stands for the “T Helper cells,” which are derived from T-lymphocytes bearing a CD4 surface marker. Such immune responses mostly involve special cells (especially macrophages and lymphocytes) and lymphocyte-produced hormones called cytokines. Antibodies, histamine, leukotrienes, and other mediators are involved in other immune reactions.
Th2 immune response is associated with the production of cytokines such as IL3, IL4, IL5, IL6, IL13, and IL10 (which inhibits the TH1 state). Natural Killer (NK) Cells promote the Th2 state, which is considered the “normal state.” Extracellular bacteria, parasites, allergens, and toxins invoke the Th2 response.
The Th1 immune response is associated with the production of pro-inflammatory cytokines such as IL2, TNF-alpha, IL12 and especially IFN-gamma. Excessive pro-inflammatory responses can lead to uncontrolled tissue damage. Th1 responds to intracellular bacteria (such as mycoplasma and chlamydia), viruses, yeasts, and some cancers.
Th1 produces IL2, which inhibits the Th2 state; and Th2 produces IL10, which inhibits the Th1 state. So there is usually a balance of Th1 and Th2 immunity, poised to address any biological “invader.”
Most researchers consider atopy (severe allergies) and CFS/ME to represent an imbalance of Th2. Viruses, yeast, intracellular bacteria, and some cancer cells invoke a Th1 response.
This graph is an elementary summary of the immune system, from McGill University:
Th17 is characterized by high levels of IL17, 21, 22, and 23. These cytokines cause tissue damage and autoimmune disorders such as MS, psoriasis, type 1 diabetes, Crohn’s disease and others. IL17 and IL23 increase the influx of eosinophils (associated with common allergies and asthma) and are associated with steroid resistant asthma.
Innate immunity (that is, immunity ingrained in everyone) constitutes the immediate reaction to “an invader,” even before T-cells, B-cells, and cytokines can react. This is mediated by antibodies to previous invaders, macrophages, NK cells and dendritic cells. Now back to the conference …
Dr. Susan Levine (New York City) began the Immunology Section with a CFI sponsored study of “Allergy-Related Signatures.” They measured allergy-associated immune or inflammatory molecules in 293 PWCs, which demonstrated an interesting division between persons with recent onset of CFS/ME and those with a longer duration of illness. Patients with less than 3 years duration had higher levels of inflammatory (Th2) molecules such as IL4, IL6, IL13, IL10, IL17A, and CCL5, and there was a tend toward lower eotaxin levels (eotaxin stimulates eosinophil migration). The short duration subset also had increased allergy-related molecules such as IL1, IL1RA, IL8, TGFa, etc. The authors suggested that an IL4 inhibitor such as montelukast/Singulair might be more effective for allergy symptoms in PWCs with a short duration of illness.
This duration-related phenomenon was echoed by Dr. Mady Hornig (Columbia University) who also found pro-inflammatory cytokines (IL4, IL10, IL23, IL2RA) increased in PWCs ill less than 3 years; but GMCSF, IL12p2O, and eotoxin were all reduced. IFN-gamma was markedly increased (OR=117, whereas an OR of >6 is considered very high), which correlates highly with cognitive impairment (OR=67).
In the cerebrospinal fluid of these subjects IL2B, IL27A, TNFa, and IFNg (Th1-related) were reduced, as were IL4 and IL10 (Th2-related), but eotaxin was increased.
[Ed.Note: We can conclude that inflammatory cytokines and allergic symptoms are most likely to be found in PWCs with less than 3 years of illness duration. Does that mean that after 3 years PWCs would be less likely to respond to immune modulators?]
Dr. Montoya’s group at Stanford has also been examining cytokines and has concluded that of 51 molecules studied, 15 of them can distinguish cases from control cases, raising the possibility of a CFS/ME marker. They also noted that higher levels of cytokines are associated with increased severity.
[Ed.Note: We typically see a lot of cytokine and immunology studies at each conference, probably because cytokines are thought to produce many of the symptoms of CFS/ME and FM. Sadly, there are few interventions for reducing cytokine levels or modifying immunity. Many more studies were presented but added little to our current understanding, in my estimation.]
Virology / Infections
Dr. John Chia is an infectious disease specialist and pathologist from Lomita CA. He and his son contracted CFS/ME and were found to have enterovirus infections in their stomachs (see his article in the Journal of Clinical Pathology, Jan 2009). After treatment with a Chinese herbal called oxymatrine (there is no other known therapy for EV) both have recovered and stayed well. Dr. Chia reported to us on further EV studies from his lab. To demonstrate the infectiousness of EV, Chia injected the lysate (Osterized tissue or homogenate) from 24 EV-positive human stomach biopsies into immune deficient SCID mice. When the mice were later sacrificed, 13 out of 20 were positive for EV in their spleens, but only 1 of 10 control mice was positive. However, Chia could not culture the virus from any of the spleens suggesting that although the infection could be transferred, incomplete viruses were formed in the receiving mice.
In a second study, Chia obtained pathology specimens from 27 women with CFS/ME who had undergone total hysterectomy or salpingo-oophorectomy for chronic pelvic pain. 24 of the 27 specimens stained positive for EV, whereas none of 15 healthy control specimens were positive. Three SCID mice were injected with lysate from EV-positive specimens and the mice were sacrificed at either 2 or 5 weeks afterward. Spleens and fallopian tubes stained positive for EV at both 2 and 5 weeks, although spleen stain was less obvious at 5 weeks. Western blot studies of all mouse fallopian tubes demonstrated enteroviral proteins.
[Ed.Note: Dr. Chia is making a strong case for enterovirus as a common trigger for CFS/ME, and these studies imply that the infection is transferrable. I found it interesting that chronic pelvic pain was localized to the infected fallopian tube in his patients, and that surgery relieved the pain. Sadly, no one else has taken on the task of confirming Dr. Chia’s studies. Also there is no known antiviral therapy for EV – just an ill-tolerated herbal preparation. Hopefully someone with Chia’s expertise will investigate this further and confirm these important findings!]
Parvovirus B19 (a very common single stranded DNA virus that causes of Fifth Disease in children and infectious arthritis in adults) was studied by Santa Rasa (research assistant at the Kirchenstein Institute of Microbiology and Virology, Latvia). She reported that serological studies for PB19 were positive in 40% of 190 patients with CFS/ME, compared to only 15% of controls. She then used nested PCR to look for PB19 viral DNA in plasma. 21% of PWCs and 2% of healthy controls were positive by this technique, but fever, lymphadenopathy, and neurocognitive problems were more common in subjects who were PCR-positive.
[Ed.Note: Add Parvovirus B19 to the growing list of infectious agents that can trigger and/or perpetuate CFS/ME. Unfortunately, there is no known treatment for PB19. One paper has suggested that intravenous gamma globulin may be effective. Because the treatment is unsure and terribly expensive, Dr. Montoya recommends treating only patients who have positive IgM serology or positive PCR for PB19.]
Exercise Testing
Exercise testing and the test-retest exercise protocol played a large role in this conference for some reason. Since the earliest days of Chronic Fatigue Syndrome research we have used cardio-pulmonary exercise testing (CPET) to prove that persons with CFS/ME and FM have relatively preserved strength but do not have the energy or stamina of normal healthy controls. Furthermore, this is not due to deconditioning alone. Cheney and I showed many years ago that the “fright or flight” response to maximal exercise was deficient in patients, which was later confirmed by Gold, et alia, at the NIH. More recently, Snell, VanNess and Stevens have taught us that when the CPET is repeated 24 hours later that PWCs – but not normal healthy controls – show a decrement in performance due to post-exertional malaise.
Betsy Keller PhD (Ithaca College) began the Exercise Section with a wonderful review of cardio-pulmonary exercise testing, and emphasized that a single CPET fails to demonstrate functional impairment in about 20% of subjects. Two serial CPET’s, however, revealed functional impairment in at least 95% of subjects. In her lab the aerobic work capacity (VO2 max) decreased by >13%, with 23 out of 42 subjects (55%) decreasing at least 6%. Similarly the aerobic capacity at anaerobic threshold (VO2 at AT) was decreased significantly on the second CPET. This decrement from Test 1 to Test 2 is attributed to post-exertional malaise, a significant phenomenon in PWCs, but much less common or severe in any other disorder. Thus, an abnormal Two-Step CPET is prima facie evidence of severe impairment (that is, disability).
Mark VanNess (University of the Pacific) took the Test-Retest Protocol further by examining ventilation (breathing) during these studies. He provided evidence of diminished ventilation during exercise testing in the patients, and even more difficulty with ventilation on Test 2, compared to healthy controls (who actually did better on Test 2!). This could only be explained by diminished ventilator muscle function during post-exertional malaise or reduced ventilator “neural drive,” but cannot be explained by deconditioning. Thus skeletal (and ventilatory) muscle fatigue likely contributes to the phenomenon of post-exertional malaise seen in CFS/ME.
Keller’s group also measured cytokines before and after exercise. They concluded that TNFa, IL1, IL6, IL12, and IL17 were not significantly affected by exercise, but IL2, IL1RA, IL8 and MCP-1 were all much lower in the patients than controls after exercise.
Both exercise groups looked at submaximal exercise testing and did not find it helpful in discriminating patients from controls.
Vermuelen (CFS/ME Centre Amsterdam) took things one step further by measuring cardiac output during the CPET. His group was then able to calculate the amount of oxygen extracted from the blood by muscles. In women, the peak oxygen extraction was 10.83 in CFS/ME, 11.62 in the chronically fatigued patients, and 13.45 ml/dl in healthy women. Similar for men. Cardiac output was actually increased during exercise for PWCs. This low peak oxygen extraction in the face of increased cardiac output is distinctly abnormal and must indicate a metabolic cause. Since lactate production after exercise is lower in PWCs than controls, Vermuelen suggests that there is a down regulation of carbohydrate metabolism.
Drs. José Alegre and Jesus Castro (Chronic Fatigue Group, Universidad Autónoma de Barcelona) studied endocrine effects of maximal exercise. They reported — as we did years ago – that cortisol was reduced in many PWCs after maximal exercise, which may explain why these patients do not adapt adequately to stress.
Prognosis
For years I have maintained that lifespan is not affected by CFS/ME, and that there is no increased risk of cancer in our patients. Also, early studies in our office and by the CDC showed that 50-60% of cases improved significantly over time. I may have to revise these opinions based on these papers:
Dana March (Chronic Fatigue Initiative) extracted information from a longitudinal database of 1430 patients with CFS/ME, of whom 67% completed a survey. Abrupt onset of illness was reported by 52% of respondees, and they experienced only modest improvement over time. However, 35% reported at least one remission (mean 2.5 remissions). These remissions lasted a mean of 199 weeks ( + 178), or a median of 52 weeks. Interestingly, the more severe cases were most likely to report improvement or remission!
PWCs reported a mean of 3.5 + 2.2 co-morbid conditions, of which the most common were fibromyalgia (61%), depression (47%), anxiety (39.7%) and hypothyroidism (35%)
The “cause of death” reported was cancer in 16% (4 times the background rate), cardiovascular disease in 19% and suicide in 19%. However, half the cancers were due to skin cancers. Nine individuals had multiple cancer diagnoses.
Lastly, Dr. Rajeevan (Centers for Disease Control, Atlanta) described telomere shortening in PWCs. This deserves a brief explanation. In your cells the DNA is twisted or braided like a cruller, and at both ends are stringy ‘fingerlets’ called telomeres. (I think of this like a horizontal stalk of broccoli, with the “head” of the broccoli representing the telomeres.) With each division/multiplication of your cells, the telomeres grow shorter and when they are about gone the cell dies (called apoptosis). Telomeres are known to be shortened by stress and oxidation radicals, among other things. Dr. Raj noted increased telomere shortening in PWCs and increased TERT (TElomere Reverse Transcriptase, a gene that is less well methylated in patients) in PWCs, both of which have been associated with aging.
[Ed. Note: regardless of the data, these findings are quite different from my 28 years of experience.]
Pediatrics
Dr. Peter Rowe (Johns Hopkins) presented several diverse papers on adolescents with CFS/ME. Studying the impact of CFS/ME on 46 adolescents and young adults, he concluded that quality of life was poorer than children with cystic fibrosis, epilepsy, diabetes, sickle cell disease and even renal transplant . This was based on self reports on various surveys of life quality.
On the other hand, Katherine Rowe (no relation to Peter; she’s from Melbourne AU) reported that only 27% of her adolescents experienced depression, whereas the prevalence is 15-20% in the teen population. Depression at baseline had no effect on recovery from Pediatric CFS, with 65% of Rowe’s patients recovering in 5 years, and 88% within 12 years. (Rowe defined “recovery” or “well” as feeling 7/10 to 10/10 compared to normal. The mean report, however, was 9/10.) So the prognosis for adolescents seems better than the prognosis for adults.
Drs. Seiki Tajima and Terahusa Miike (Kobe, Japan) posited that the Japanese are a sleep-deprived people and that lack of sleep leads to CFS/ME in children and adolescents. (A previous paper identified that 83% of children who are chronically absent from elementary school met criteria for CFS/ME.) Tajima’s group simply instituted a sleep education program in selected elementary schools which markedly improved student absenteeism and reduced the incidence of CFS/ME.
Joint laxity (Ehlers-Danlos Stigmata or EDS) has been a favorite subject for Dr. Peter Rowe, who introduced this concept to us years ago. The prevalence of EDS is up to 40% in healthy adolescents, but more than 60% in PWCs. The condition is frequently associated with other musculoskeletal disorders such as kyphosis, lumbar lordosis, and reduced ankle dorsiflexion.
Rowe’s first presentation dealt with impaired range of motion in 48 pairs of adolescents who were matched for degree of flexibility. Rowe found that – compared to hypermobile healthy adolescents – PWCs had reduced ankle dorsiflexion (< 95 degrees), markedly reduced passive leg raising ( egg and wheat. The diagnosis is made by avoiding the offending agents and noting improvement in symptoms. There is no treatment except avoidance. To make a long story short, Rowe studied 55 PWCs between the ages of 10 and 23, of which 14 ( = 25%) had milk protein sensitivity.
Brain Research
Marcie and Mark Zinn (Stanford) presented two papers on quantitative EEG technology. They sought PAF (Peak Alpha Frequencies) in the electroencephalograms of 50 PWCs and 50 controls. PAF is associated with memory, attention, and learning, they explained, and increased PAF occurred in 58% of patients (especially the frontal lobes and central and midline cortex). High levels of PAF correlated with higher fatigue score on the MFI-20 (Multidimensional Fatigue Inventory) and the FSS (Fatigue Severity Scale). The pattern of activity suggested a hyperregulation of thalamo-cortical circuits and is associated with an interruption in goal-directed behavior and reduced executive skills such as alertness, attention, information storage and retrieval, and temporal organization.
Using a technique called eLORETA, the Zinn’s also demonstrated increased delta activity (sleep waves) in the frontal and limbic regions, especially over the left hemisphere. (Language and other cognitive functions are left hemisphere dominant, even in “lefties.”) This would lead to disruptions in information transfer in the brain (that is, storage and retrieval), destabilization of ascending arousal mechanisms, deficits in language production and syntax, and inhibition of beta and gamma (awake) waveforms. Beta-2 was also reduced in the medial frontal gyrus and paracentral areas, which would explain motor deficits (delayed reaction time, psychomotor slowing, and slow processing), sensory ataxia (poor balance, tandem stance and Romberg exams), and pain hypersensitivity.
[Ed. Note: Much of this is not new. Dr. Myra Preston, who worked in our office, noted in the late 1980’s that our patients were awakened by alpha (awake) waves when they tried to sleep, and generated delta (sleep) waves when they were trying to stay alert. Serendipitously we learned that those delta waves could be suppressed by stimulant medications such as Ritalin, Adderall, and later Provigil. By the early 1990’s she had described and patented a classical “EEG signature” that could discriminate CFS/ME from other disorders. What is exciting about the Zinn studies is that they can physically and scientifically explain many of the neurocognitive issues that plague our patients.]
Susan Cockshell (University of Adelaide, AU) reported that 89% of PWCs complain of cognitive dysfunction, and she examined this dysfunction by four different methods. First, a review of the literature (meta-analysis) revealed the major deficits to be reduced reaction time, reduced attention, slowed motor function, poor memory, visuo-spatial deficits, reduced cognitive flexibility or reasoning, global function, and verbal/language abilities – in that order.
Next her group examined “effort,” or more aptly whether patients were malingering, benefiting from secondary gain, or just unable to perform. Examining 54 PWCs and 54 controls with the Validity Indicator Profile Test, she found that 93% of patients and 93% of controls were compliant. Thus, she found no evidence of malingering or secondary gain.
The third study correlated cognitive deficits with tests of fatigue, sleep quality, anxiety, depression, and every day functioning. Long and short: cognitive deficits were unrelated to motor problems, depression, anxiety, fatigue, or sleep deprivation. Therefore, the main cognitive deficits in PWCs appear to be due to a slowing in information processing speed.
Lastly, her group studied the correlation between self-reported cognitive problems and neurocognitive testing. Surprisingly, complaints did not correlate with the cognitive tests for either patients or controls! However, it took 7 hours for controls to recover (fatigue) from the testing, while PWCs took an average of 57 hours to recover.
[Ed. Note: This elegant study once again confirmed what we have known clinically for years: slow information processing and retrieval explain the bulk of cognitive deficits; patients are not malingering or “gaming” the tests; and current cognitive testing is relatively insensitive in picking up the subtle impairments caused by “brain fog.” ]
A poster presentation was of interest to me. Sorenson, Jason, et alia (Loyola and DePaul Universities, Chicago) reported that Brain-Derived Neurotrophic Factor (BDNF) is decreased in Chronic Fatigue Syndrome. BDNF is protein involved in the maintenance and maturation of neurons in the brain and body, so a lack of the substance would reflect an ineffective repair mechanism. Comparing levels of BDNF in PWCs, persons with multiple sclerosis (MS), and healthy controls, BDNF was similar in CFS/ME and MS, but 25% lower than in the healthy controls.
Severely Ill
One entirely new topic this meeting was “The Severely Ill,” presented by myself and Irma Pinxsterhuis and Elin Strand (CFS/ME Unit, Oslo University Hospital, Norway). This population of severely affected patients is mostly invisible, ignored, and underserved, but may represent up to 25% of all persons with CFS/ME and FM. There is very little written about the subject, and a Medline search revealed only 13 apropos papers on the subject, out of over 5000 articles on Chronic Fatigue Syndrome, ME, and FM.
I define these patients as “mostly housebound and recumbent most of the day except perhaps for necessary medical or religious reasons.” On scales of fatigue and quality of life they rank lower than persons with crippling rheumatoid arthritis, severe emphysema, kidney failure, and even terminal congestive heart failure.
Experience demonstrates that in addition to the core symptoms of exhaustion after minimal activity, pain, severe neurocognitive impairment, and sleep disruption, these patients experience added and more severe co-morbidities including orthostatic intolerance, irritable bowel and bladder, multiple chemical sensitivities, gut motility and sprue-like disorders, abdominopelvic pain, air hunger, and numerous neurologic disorders such as myoclonus, paresis, dysesthesias, and “blackouts.” By virtue of being bedridden they are susceptible to delayed onset muscle soreness, functional malnutrition, massive deconditioning, postural hypotension, pressure sores, contractures, even osteoporosis and deep venous thromboses. Studies have also shown that the severely ill have a low cardiac output, a higher incidence of diastolic dysfunction, and functional volume depletion with low plasma volume and RBC mass.
Discussions during the three hour seminar, held March 20th, concluded that there are no firm guidelines on the management of such patients, but that we should treat symptoms first and then comorbidities before employing any investigational or unconventional therapies. Special considerations include:
avoid attributing new symptoms to CFS/ME/FM (rule out organic causes)
minimize medication use
‘start low and go slow’ with medications
consider liquids, crystals or gummies for medication delivery instead of just pills
regular infusions of IV fluids and volume expanders may be helpful for orthostatic intolerance
regularly monitor vital signs including intake/output, supine and sitting/standing BP, pain levels
keep a diary or journal of daily activity to monitor changes over time
engage the patient in wanting to get better and participating in the treatment process
establish a consistent pattern of activity and rest
establish a regular sleep routine
cautiously challenge misconceptions
challenge fearful thoughts (perhaps about activity, the future, etc.)
address the management of setbacks, which are to be expected
consider supportive counseling
encourage getting out of bed, or at least sitting upright in bed
consider home physical therapy (passive range of motion > active range of motion > light resistance > light
aerobic activity)
consider occupational therapy to learn energy conservation
include alternative therapies (especially mediation/relaxation, self-hypnosis/imagery, massage,
balneotherapy, and aromatherapy)
most important is to provide adequate nursing, help, and support so that the patient feels secure and tranquil
Caretakers are encouraged to build a “team” to include a primary medical doctor, an emergency strategy, intermittent nursing care, and transportation; perhaps a tutor, social worker, dietician, or speech language pathologist. Don’t forget to seek governmental benefits and obtain legal documents (power of attorney, advanced directives).
Remarkably, the prognosis for recovery is better than might be expected. Reports of three adolescents with severe CFS/ME describe virtually total recovery following intensive home-based (1) and inpatient (2) support (see Burgess and Chalder, BMJ Case Reports, 2011). Hill, Tiersky and Natelosn (Arch Phys Med Rehab, Sep 1999) reported on 23 severely ill adults who were followed for about 4 years. During that period of time 1 recovered, 9 improved, and 13 remained ill. VanAbbema et alia (Disabil Rehab 2011) described the effectiveness of a multidisciplinary program for 87 persons with severe FM, of which 34 made ‘clinically significant improvement in quality of life.’
Good resources for more information include the IACFS Primer for Clinical Practitioners (www.iacfsme.org/Home/Primer/tabid/509/Default.aspx), and Emily Coleridge’s “Severe ME/CFS: A Guide to Living” (available at www.ayme.org.uk ). Ms. Coleridge herself succumbed to severe ME.
It is our hope that this seminar will encourage more interest in this large and underserved population of patients.
Biobanking
Several “biobanks” have started collecting specimens of blood, bodily secretions, and even pathological specimens. These biobanks store “patient material” for years and even decades, then provide specimens to qualified researchers. This permits rapid access to specimens as well as longitudinal studies, which should help us to understand Chronic Fatigue Syndrome much more quickly than ever before. Such biobanks are maintained by:
- The CFIDS Association
- Stanford University
- Nova Southeastern University
- Chronic Fatigue Initiative
- NIH Multicenter Study (Columbia University)
- Simmaron Research (Nevada)
- Centers for Disease Control (Atlanta)
- UK ME/CFS Biobank
Lapp, Kalns, Springs, and Thompson presented a poster on salivary markers in CFS/ME. Based on an initial study of banked saliva, a unique compound was isolated from PWCs compared to healthy controls. We enlisted 40 patients and 20 controls to collect saliva and confirm the pilot study. Once again a unique compound was found to be twice as concentrated in PWCs than controls, suggesting a possible biomarker for the disorder.
I would like to take this opportunity to thank the many patients who have participated in these biobank projects, especially the CFIDS Association Biobank and the many research studies that we have sponsored at Hunter-Hopkins. It is frequently a sacrifice to join such projects. Participants not only provide specimens, but also large amounts of time and effort in order to benefit their fellow sufferers. Thank you all very much!
Komaroff Summary
Dr. Anthony Komaroff (Harvard Medical School) concluded the meeting with his usual excellent summary of key papers. Although he did not translate any of the scientific presentations into clinically useful strategies, he did conclude that robust evidence established CFS/ME as a real illness involving the brain and autonomic nervous system, the immune system, energy metabolism, oxidative and nitrosative stress. He pointed out that currently there is no recognized therapy or cure for CFS/ME or FM, but tricyclic antidepressants, NSRIs, and anti-epileptics like pregabalin have been shown effective against pain. He cited ongoing studies from Norway (rituximab) and Stanford University (valganciclovir) that are hopeful. His final comment to conference attendees was, “the illness is not simply the expression of somatic symptoms by people with a primary psychological disorder.
How Will This Information Change The Way I Practice?
I always end this biennial report with a statement on how this meeting will change the way I practice. As I suggested in the introduction, there was not much new with respect to treatments. The goal at Hunter-Hopkins Center has always been to provide the best and most current therapies for our patients. There are six goals that come out of this meeting, however:
- We need to work harder on patient education and support
- I will check carnitine levels more regularly (low carnitine is associated with increased cognitive difficulty and may be a surrogate marker for IL17)
- I will encourage more patients to undergo Two-Part Cardio-Pulmonary Exercise Testing
- I will encourage more patients to follow NK Cell Activity as well as cytokine panels (INFg, IL6, IL4, IL10) as a measure of management success
- I will be more vigilant for evidence of Parvovirus B19 and Enteroviral infections
- We need to develop a “toolkit” for those providers who see the severely ill patients.
______________________________________________________________
Charles W. Lapp, MD, Director
Hunter-Hopkins Center, P.A., Charlotte, North Carolina
March 30, 2014
The above information reflects the personal opinions of the author only, and is not meant to be an exact or exhaustive review of the IACFS conference. The author would appreciate comments or corrections.
This material is copyrighted, but may be reprinted with permission of the author and with appropriate credit. Contact drlapp@drlapp.net. (© 2014)