- Home
- Research
Hunter-Hopkins Center maintains a multi-specialty consultation and research site in Charlotte, North Carolina, and conducts clinical trials, nutraceutical trials, and clinical research. These studies are performed independently or collaboratively with other researchers throughout the world.
For more information, contact Wendy Springs CCRP, our research coordinator, at the Hunter-Hopkins Center.
We’re actively looking for ways to defeat CFS, ME and FM.

Hunter-Hopkins Center is actively involved in CFS, ME and FM research in the United States and worldwide. This insures that our patients will receive accurate, up-to-date information and treatments.
Currently open or ongoing studies:
Ampligen. We have received approval from the Federal Drug Administration to administer Ampligen to seriously ill patients with CFS. For more information, click the Ampligen tab above.
Long Covid. Ampligen has been shown effective in treating a virus closely related to Covid, known as SARS or SARS-CoV-1. Also, many persons with Long Covid develop a CFS-like illness. Another current study treats persons with persistent Covid (“Long Haulers”) with Ampligen. We are hopeful this will reduce the symptoms of Long Covid and/or the CFS-like symptoms.
Multisite Clinical Assessment of ME/CFS (MCAM). This study was initiated by the Centers for Disease Control in 2011 and HHC is one of seven expert clinical centers chosen to participate in this longitudinal study designed to document a comprehensive picture of CFS patients identified in multiple clinics and to describe the approach that experts use to diagnose and manage their patients.” This study officially closed in 2019, but data is still being compiled and medical papers prepared.
Ampligen is a step toward effective treatment.

There is no known cure for CFS, but there is hope for an effective treatment with Ampligen.
Ampligen (generic name rintatolimod)is an experimental anti-viral and immune modulatory drug manufactured by AIM Immunotech of New Jersey. In a double blind placebo controlled study performed from 1990-1992, approximately 80% of the 45 CFS subjects improved on the drug, and 50% of subjects improved significantly. Many were able to return to work again after a few months of treatment! A similar study conducted in Belgium reported results that parallel the 1990-1992 U.S. study. Several hundred subjects with CFS have been treated so far with Ampligen, and thousands of doses of the drug have been administered safely.
Does it Work?
The Medical Director of AIM Immunotech, Dr. David Strayer, presented preliminary data on Ampligen to an international group of scientists (7th Annual AACFS Research and Clinical Symposium, Madison, Wisconsin, October 2004). In summary: Dr. Strayer reported on a Phase III randomized, double blind placebo controlled crossover study of 234 subjects treated with parenteral Ampligen 400 mg twice weekly for 40 weeks. Demographics were similar in both groups. The dropout rate was slightly increased (24 v 16) as were the Serious Adverse Events (16 v 8.0) in the treatment group compared to placebo, but the differences were not statistically significant (p > 0.10).
Exercise duration in the treatment group was 16.1% greater than placebo (in completers) and 15.2% greater than placebo in all participants (intent to treat analysis, p 0.05). These increases in exercise duration were over twice the minimum considered medically significant (6.5%). Maximum oxygen utilization was markedly improved in treated (6.07) versus placebo (0.58) subjects.
There were no significant adverse events or significant abnormalities in laboratory parameters. Ampligen treatment in this debilitated population of CFS patients resulted in a medically and statistically significant improvement in the primary endpoint, exercise treadmill duration, compared to placebo. Ampligen may be the first drug to demonstrate safety and effectiveness in the treatment of CFS.
Availability
As of March 19, 1997, Dr. Lapp received FDA approval to administer Ampligen on a “cost recovery basis,” which means that the patient must bear the cost of the drug, safety monitoring and administration. So far, more than 60 persons with CFS (PWCs) have taken Ampligen as part of this cost recovery program, more than 12 of whom had significant improvement, and many others have improved.
The Treatment
Ampligen is only available as an intravenous drug. Typically 400mg is administered twice weekly over the course of about one hour. Most subjects experience mild flu-like feelings for several hours after the infusion. While research demonstrates that 6 months of therapy can produce a significant response, we generally recommend at least 12 months of therapy. Persons who have been ill for a long time are well advised to consider at least 18 months of therapy. In our cohort of subjects we have seen obvious improvement in as little as 24 weeks, but the average treatment period was about 70 weeks.
Restrictions
There are several restrictions that apply to subjects receiving Ampligen treatment. The subject must be 18 to 70 years old and very ill. Female subjects with child-bearing potential are required to use regular birth control. While most medications may be continued, subjects may not use immune modulating drugs (such as gamma globulin, steroids, interferon, etc.), anti-viral drugs (like Zovirax, Famvir or Valtrex), non-steroidal anti-inflammatory drugs (such as aspirin, Advil, naprosyn, etc.), or any other experimental medication. Other restrictions may apply, and each subject is considered individually.
It is highly recommended that persons receiving Ampligen transfer residence to Charlotte temporarily, in order to minimize travel. We have found that more than an hour of traveling to and from the office is difficult for most subjects, and can undo the benefit of Ampligen. Our staff can recommend rental apartments nearby.
Costs
The cost of Ampligen is about $400 per 400mg dose, or $3200 per month. Infusion costs, medical visits, and laboratory add approximately $1200-1400 monthly, thus the total cost may exceed four thousand dollars per month. Some insurers will reimburse medical visits, lab, and infusion costs; and an occasional insurer will reimburse the cost of Ampligen. Medicare does not cover any expenses for investigational therapies.
More information
Ampligen has been available on a cost recovery basis in the USA, Belgium, and Canada, but only two US clinics currently provide this drug worldwide. Hunter-Hopkins was the first to offer such a program in the USA, and has been continuously providing Ampligen therapy since FDA approval in 1997.
Dr. Lapp has been treating patients with Ampligen since 1988, through FDA sanctioned research protocols.
Interested individuals should contact our Research Coordinator at (704) 543 9692 for more information or to register for treatment. You may also wish to consult the manufacturer’s website at http://www.hemispherx.net/ .
Results of a Phase III AMP-516 Study were published in March 2012, and can be viewed HERE.
Computerized CNS Vital Signs™ helps in CFS, FM diagnosis.

A Comparison of Simple Office Screening and a Computer-Based Test Battery to Quantify elements of Cognitive Dysfunction in Persons with Chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM)
Charles W. Lapp*, MD, Rebekah S. Smith*, Wendy Fallick*, Tom Gualtieri, MDº
* from the Hunter-Hopkins Center, P.A., Charlotte, North Carolina
º from North Carolina Neuropsychiatry, P.A., and CNS Vital Signs™, Chapel Hill, North Carolina
Chronic Fatigue Syndrome (CFS) is a disorder characterized by debilitating fatigue, recurrent flu-like symptoms, sleep disruption, and neurocognitive difficulties, most commonly memory loss, reduced attention, and slow processing [Ref 1]. Fibromyalgia (FM) is a related disorder characterized by chronic widespread pain, but also fatigue, sleep disruption, and cognitive difficulties. It is difficult for medical practitioners to quantify cognitive dysfunction unless they are trained in neurocognitive testing.
In 2003, a computer-based program called CNS Vital Sign™ was released, and purports to measure these parameters using adaptations of simple, standardized, psychometric tests [Ref 2]. The program is inexpensive and easy for untrained assistants to administer. It generally requires less than 30 minutes to complete, and provides immediate on-site reporting of results. It is, therefore, well suited for screening, obtaining baseline levels, or following cognitive changes over time.
The CNS Vital Signs™ battery addresses the most important cognitive domains: Attention, Memory, Motor Control, Psychomotor Speed, Reaction Time & Information Processing Speed. The screening battery includes seven selectable tests (Verbal Memory Test, Visual Memory Test, Symbol Digit Coding Test, Finger Tapping Test, Stroop Test, Continuous Performance Test and the Shifting Performance Test) covering five cognitive domains. All of these tests are publicly available and widely accepted measures of their respective domains. CNS Vital Signs™ simply provides a convenient computerized platform for administering and grading these tests. For ease of interpretation, CNS Vital Signs™ also calculates five parameters based on the results of testing (Memory, Mental Speed, Reaction Time, Attention, and Cognitive Flexibility) and grades the subject in each category as Above Average, Average, Below Average, or Well Below Average [Table 1, Overview of Domain Scores].
In order to minimize patient fatigue, we limited this study to 4 tests: Verbal Memory Test, Visual Memory Test, Symbol Digit Coding Test, Finger Tapping Test [ Table 2, CNS VS Domains].
The purpose of this study was to compare results from this computer-based program in patients with CFS and/or FM (CFS/FM) to two simple office screening tools (Serial 7 Subtraction and the Digit Span Test), normative data, the DSM-IV based General Assessment of Functioning, the Modified Karnofsky Score, and three standardized instruments (the Medical Outcome Survey Short Form-36 Scores, Hospital Depression and Anxiety Scale, Fatigue Scale, and a Visual Analog Pain Scale) [Table 3, Screening Tests and Instruments].
Methods
Subjects were derived from new and established patients of our tertiary medical clinic, which specializes in CFS and FM. Subjects were not selected or excluded in any way except that they benefited from cognitive testing as part of their management. The data was retrospectively compiled from questionnaires and instruments that are routinely collected from patients in our practice, and each subject provided a general release for the use of anonymous data in the chart.
Age and gender-matched controls were derived from a database of anonymous normal healthy subjects maintained by Dr. Gualtieri.
Statistical analysis was performed using standard statistical software [Ref 3].
Results
Using the t-test for each independent sample, subjects with CFS / FM fared much worse in all of the CNS Vital Signs survey (verbal memory (VBM), Visual Memory (VIM), finger tapping (FTT), and Symbol Digit Coding (SDC) ) than normal controls [see Table 4, Subjects versus Controls].
A number of conclusions can be drawn from examining the intercorrelation of variables using ANOVA analysis of the data [Table 5, Intercorrelations of Variables].
Serial 7 Subtraction (SSS)
Serial 7 Subtraction correlated moderately well with the Finger Tapping Test, Symbol Digit Coding correct, and Mental Speed on CNS VS.
Poor performance on Serial 7 Subtraction was moderately correlated with a low Karnofsky Score, but was not affected by anxiety or depression as measured by the Hospital Depression and Anxiety Scale.
The Karnofsky score correlated well with the Energy Subscale of the Medical Outcome Survey as well as the Finger Tapping Test and Mental Speed.
Digit Span
Digit Span forward and backward correlated moderately with Verbal Memory and overall Memory as expected, since Digit Span is thought to reflect both memory and attention.
Digit Span did not correlate with the Karnofsky Score nor to anxiety and depression as measured by the Hospital Depression and Anxiety Scale, suggesting that it is relatively independent of physical illness and psychological stresses.
Instruments
Depression and anxiety (from the Hospital Depression and Anxiety Scale) correlated moderately well with Symbol Digit Coding, the Finger Tapping Test, and Mental Speed. This is understandable since these psychological disorders are associated with both mental and psychomotor slowing.
The Medical Outcome Survey Short Form 36 correlated well with GAF, the Fatigue Scale, and the Karnofsky Scale, thus distinguishing itself as an excellent measure of impairment due to psychological factors, fatigue, and general wellness. The use of the Medical Outcome Survey for predicting impairment in Chronic Fatigue Syndrome has been previously established by Komoroff, and confirmed by our data as well [Ref 4].
None of the instruments (MOS SF-36, GAF, Fatigue Scale, Karnofsky) except the Pain Scale correlate at all with cognitive function as measured by CNS VS. Thus, CNS VS provides another and unique dimension for categorizing persons with CFS / FM.
The Pain Scale correlates inversely with Symbol Digit Coding, Mental Speed, and the general Karnofsky Score as expected, because pain is well known to interfere with concentration or attention (Symbol Digit Coding and Mental Speed), mobility, sleep, and general well-being (Karnofsky). Interestingly, increased pain does not seem to interfere with fine motor activity (Finger Tapping Test).
Conclusions
Simple office screening tests such as Serial 7 Subtraction and the Digit Span test correlate with and are indicative of cognitive dysfunction, based on this study. In cases where these screening tests are abnormal, CNS Vital Signs™ is able to capture and quantify abnormalities in specific domains such as verbal memory, visual memory, psychomotor slowing, and mental speed. Furthermore, this study shows that CNS Vital Signs™ measures elements of cognitive dysfunction that are not reflected in other frequently used instruments such as the Karnofsky Scale, Fatigue Scale, Medical Outcome Survey, or even the Global Assessment of Function.
CNS Vital Signs™ is inexpensive and simple to administer in the office, so that it can provide objective evidence of neurocognitive impairment for disability purposes, and serial testing may be valuable for following the neurocognitive response to therapy. The authors suggest that using all seven tests available on CNS Vital Signs™ allows the calculation of Reaction Time and Attention scores in addition to the domains measured in this paper. Based on previous studies of neurocognitive dysfunction in CFS / FM, it is suspected that these results would define more deficits in persons with Chronic Fatigue Syndrome and Fibromyalgia.
References
- Busichio K, Tiersky LA, Deluca J, Natelson BH, Neuropsychological deficits in patients with Chronic Fatigue Syndrome, J Int Neuropsychol Soc. 2004 Mar;10(2):278-85
- Gualtieri, T, et al., Reliability and validity of a new computerized cognitive screening battery, presented to the International Neuropsychological Society Annual Meeting, Baltimore MD, February 4-7, 2004
- Software for statistics
- Komaroff AL, Fagioli LR, Doolittle TH, Gandek B, Gleit MA, Guerriero RT, Kornish RJ 2nd, Ware NC, Ware JE Jr, Bates DW, Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups, Am J Med. 1996 Sep;101(3):281-90.
This was presented as a poster at the 7th Annual Research and Clinical Conference of the American Association of Chronic Fatigue Syndrome held October 8-10, 2004, in Madison, Wisconsin,
© Charles W. Lapp, MD, October 2004
Table 1, Overview of Domain Scores
| SCORES | NORMAL | BORDERLINE | ABNORMAL | |
|---|---|---|---|---|
| ABOVE AVERAGE | AVERAGE | BELOW AVERAGE | WELL BELOW AVERAGE | |
| WHAT THEY MEAN |
> 84th percentile |
Percentile 17-83 |
Percentile 3-16 |
The bottom 2 per cent |
| MEMORY |
How well subject is able to remember words and geometric figures. |
|||
| MENTAL SPEED | Motor speed, fine motor coordination, visual-perceptual ability. | |||
| REACTION TIME |
How fast the subject can react, in milliseconds, to complex directions. |
|||
| ATTENTION |
Oneâs ability to maintain focus and perform quickly and accurately. |
|||
| COGNITIVE FLEXIBILITY | How well the subject is able to adapt to rapidly changing directions. | |||
|
The Domain Scores are computed from the scores subjects attain |
||||
Table 2, CNS VS Domains
|
SCORES |
NORMAL |
BORDERLINE |
ABNORMAL |
|
|---|---|---|---|---|
|
ABOVE |
AVERAGE |
BELOW |
WELL BELOW |
|
|
WHAT THEY MEAN |
> 84th percentile |
Percentile 17-83 |
Percentile 3-16 |
The bottom 2 per cent |
|
MEMORY |
How well subject is able to remember words and geometric figures. |
|||
|
MENTAL SPEED |
Motor speed, fine motor coordination, visual-perceptual ability. |
|||
|
REACTION TIME |
How fast the subject can react, in milliseconds, to complex directions. |
|||
|
ATTENTION |
One’s ability to maintain focus and perform quickly and accurately. |
|||
|
COGNITIVE FLEXIBILITY |
How well the subject is able to adapt to rapidly changing directions. |
|||
|
The Domain Scores are computed from the scores subjects attain |
||||
Table 3, Explanation of Screening Tests and Instruments
| Serial 7s | Subject is asked to subtract 7 serially from 100 (i.e., 93, 86, 79, 72, 65). Subject is given one point for every correct answer. Maximum is 5 correct. |
Ref |
| Digit Span | Subject is asked to repeat 3, 4, 5, 6, and 7 digit numbers in reverse order. Score is the number of answers without error. |
|
| GAF | General Assessment of Functioning as defined in the DSM-IV. | |
| SF-36 | Short Form-36 Health Survey (New England Medical Center). Each subscale represents the subjectâs perception. 100 is the maximum score, and worse perceptions have lower numbers. |
|
| Karnofsky | This Modified Karnofsky Score is a rating assigned by the examiner, in increments of 5. 100 is normal, over 80 implies good function, under 60 implies poor functioning, 0 is dead. |
|
| HDAS | Hospital Depression and Anxiety Scale. Scores represent depression scores / anxiety scores. A score of 0-7 is normal, 8-10 is intermediate, and higher scores imply worse morbidity. |
|
| FS | Fatigue Scale. Higher score indicates more severe fatigue. Maximum is 160. |
|
| Pain Scale | This is a 0-to-10 visual analog scale. The patient is asked to mark where his typical pain would be on the scale, 0 being no pain and 10 being the most severe pain ever. |
Table 4, Subjects versus Controls
|
Group |
Controls |
CFS / FM |
||
| N = | 30 | 30 | ||
| Age | 41.73 years | 41.73 years |
t |
p < |
| VBM tot | 53.60 | 48.97 | 3.5 | 0.001 |
| VIM tot | 47.43 | 43.37 | 3.33 | 0.002 |
| R Taps | 58.93 | 46.57 | 3.99 | 0.000 |
| L Taps | 55.70 | 45.23 | 3.63 | 0.001 |
| SDC corr | 56.57 | 46.67 | 3.00 | 0.004 |
| SDC err | 1.5 | 0.63 | 1.70 | 0.095 |
| MEM | 101.03 | 92.13 | 4.34 | 0.000 |
| MS | 171.11 | 138.37 | 4.23 | 0.000 |
Table 5, Intercorrelations of Variables
|
SSS |
DSf |
DSb |
SF-36 |
VBM |
R Tap |
L Tap |
SDC |
SDC |
HDAS |
HDAS |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
|
VBM |
0.38 |
0.45 |
0.51 |
0.06 |
1 |
||||||
|
T Tap |
0.46 |
0.35 |
0.24 |
0.08 |
0.30 |
1 |
|||||
|
L Tap |
0.48 |
0.36 |
0.24 |
0.03 |
0.03 |
0.96 |
1 |
||||
|
SDC |
0.47 |
0.20 |
0.37 |
0.04 |
0.47 |
0.54 |
0.48 |
1 |
|||
|
SDC |
0.07 |
0.06 |
0.25 |
0.27 |
0.15 |
-0.08 |
-0.09 |
-0.10 |
1 |
||
|
MEM |
0.34 |
0.31 |
0.52 |
-0.19 |
0.79 |
0.30 |
0.33 |
0.67 |
0.10 |
||
|
MS |
0.54 |
0.35 |
0.32 |
0.06 |
0.43 |
0.95 |
0.92 |
0.77 |
-0.10 |
||
|
KPS |
0.39 |
0.24 |
0.09 |
0.42 |
0.27 |
0.48 |
0.43 |
0.37 |
0.21 |
||
|
HDAS |
-0.38 |
-0.42 |
-0.28 |
-0.05 |
-0.29 |
-0.49 |
-0.40 |
-0.41 |
0.13 |
1 |
|
|
HDAS |
-0.06 |
-0.27 |
-0.10 |
-0.09 |
-0.23 |
-0.42 -0.30 -0.44 0.01 0.70 1 |
All shaded figures indicate a correlation of r < 0.5. Dark shading indicates stronger correlation.
Key:
| SSS | Serial 7 Subtraction |
| DSf | Digit Span forward |
| DSb | Digit Span backward |
| SF-36 | Medical Outcome Study SF-36 (energy scale only) |
| VBM | Verbal Memory score, total |
| R Tap | Right handed Finger Tapping |
| L Tap | Left handed Finger Tapping |
| SDC corr | Symbol Digit Coding, correct answers |
| SDC err | Symbol Digit Coding, errors |
| HDAS d | Hospital Depression and Anxiety Scale, depression |
| HDAS a | Hospital Depression and Anxiety Scale, anxiety |
| MEM | Memory score from CNS Vital Signs |
| MS | Mental Speed score from CNS Vital Signs |
| KPS | Modified Karnofsky Performance Score |
Updated April 2017
We’ve been committed to a cure from the beginning.

Closed or completed studies:
Long Covid Study (Preliminary). In July 2022, AIM Immunotech (manufacturer of Ampligen) reported positive preliminary pilot study data from its ongoing Expanded Access Program (“EAP or “AMP-511”). This study evaluated Ampligen as a therapeutic agent for “Long COVID”. The preliminary data from this uncontrolled clinical trial found that patients reported statistically significant improvements in chronic fatigue, the ability to be more active, and post-exertional malaise after treatment with Ampligen.
Tom Equels, CEO of AIM stated, “Post-COVID conditions are debilitating, can be brutally agonizing and affect millions of people who are suffering without any viable treatment options. The positive preliminary data demonstrated in this pilot study bolsters our confidence in the potential of Ampligen as we continue to drive its development as a potential therapeutic for the treatment of long COVID. With the positive preliminary results demonstrated to date we remain, now more than ever, steadfast in our mission to bring an effective therapeutic option to those in need.”
Based in part on this early positive data, AIM is continuing to work toward filing a Investigational New Drug application with the FDA for a Phase 2 study of Ampligen for the treatment of Post-COVID conditions. The planned IND is for 12 weeks of therapy.
The first four enrolled Post-COVID patients were treated in the study. Evaluation of safety is a secondary objective in this study. The initial findings for these four enrolled patients are as follows:
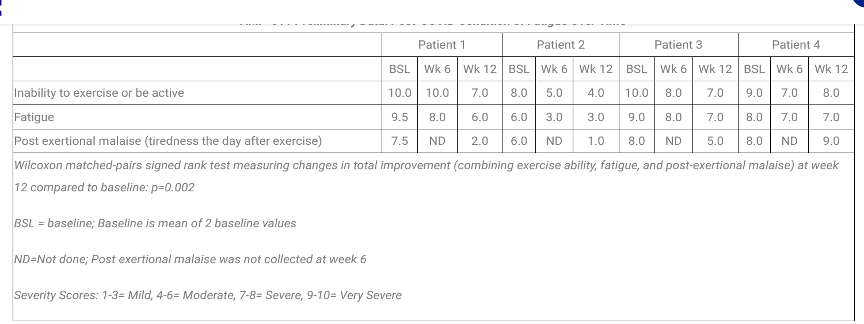
Hunter-Hopkins Center was honored to implement this study and hopes to participate in future Long Covid treatment studies.
Multisite Clinical Assessment of CFS (MCAM). Initiated by the CDC, this was a longitudinal study designed to document a comprehensive picture of CFS patients identified in multiple clinics and to describe the approach that experts use to diagnose and manage their patients. This massive study completed in 2019. Data is currently being compiled and scientific papers are being written. The first paper, “Design of a Rolling Cohort Study,” was published in the American Journal of Epidemiology in 2017 and can be found here.
Hyperion Biotechnology Saliva Study. In a pilot study, Hyperion had previously discovered a salivary peptide that distinguished PWCs from the non-fatigued population. This study sought confirmation of this potential CFS marker in 36 PWCS and 20 healthy controls. In fact, salivary peptide levels were twice as high in controls than PWCs, and there was no relationship to age, weight, medication use, presence of fibromyalgia, or time of specimen collection. Results were reported at the 2014 IACFS International Research Conference in San Francisco.
Droxidopa. We have completed (2011) a “Study to Assess the Clinical Benefit of Droxidopa in Persons with Chronic Fatigue Syndrome” for Chelsea Therapeutics. Droxidopa stimulates the production of norepinephrine, and is thought to be effective in orthostatic intolerance (such as POTS or NMH), orthostatic hypotension, and low blood pressure.
Hunter-Hopkins has completed two studies for Pfizer Pharmaceuticals to study pregabalin/LyricaTM for the treatment of fibromyalgia pain. Pregabalin is a GABA potentiator that is also useful for seizures and anxiety. This study was published in the Journal of Rheumatology (Feb 2008). To read the paper click here.
Ampligen. We also received approval from the Federal Drug Administration to participate in an FDA Phase III double-blind crossover study of Ampligen sponsored by the manufacturer, Hemispherx Biopharma (completed July 2004).
RESST Study (eszopiclone/LunestaTM). We have completed a study for Sepracor to study their new sleep medication, Lunesta, in patients with CFS/FM ( April 2005).
We have completed a study of almotriptan/AxertTM (Ortho-McNeill) for the treatment of migraine. This was a post-marketing study to determine the effectiveness of Axert for delayed treatment of migraine, which is common in CFS/ME.
LEAP Study. We have completed a Phase IV study of the Lilly product, duloxetine/CymbaltaTM. Duloxetine is a Norepinephrine Serotonin Reuptake Inhibitor (NSRI) that is expected to improve mood and reduce fibropain. Subsequent to such studies, Cymbalta was approved by the FDA for the treatment of fibromyalgia pain. More details can be found at ImmuneSupport.com.
In July 2004 we completed a pilot study of CNS Vital Sign™, a computer-based psychometric test. We believe that this test will improve the availability and reduce the cost of traditional neuropsychometric testing. CNS VS could be used to establish disability as well as follow cognitive ability over time. To view the results of this study, click the “CNS Vital Signs” tab at the top of this page.
Hunter-Hopkins completed a pilot study using the Accusplit 120TM stepometer to monitor activity in persons with CFS/ME/FM. This pedometer-like device measures one’s activity over several days, allowing us to subtype individuals (high, low, or normoactive) and follow progress over time. We found that there is a great deal of variability between pedometers (even from the same manufacturer), but a stable normoactive PWC typically takes 6000-7000 steps per day, whereas healthy controls took about 10,000 steps per day. We therefore recommend the use of a simple pedometer to monitor activity. Aim for no more than 6000 steps daily.
CFS and pregnancy. A retrospective chart review of our experience. Completed and published in the CFS Research Review. Click review.
Transfer factor. We completed a pilot study of this immunomodulatory product, in cooperation with Pro Health, Inc., and Immunosciences Laboratory (December 2002)
Stimulant therapy in CFS. A prospective study using modafinil (ProvigilTM, Cephalon Inc) in persons with CFS (completed December 2001).
Symptoms Predict the Outcome of Tilt Table Testing in CFS/ME/FM. We retrospectively studied 104 persons with CFS/ME or FM in our Cardiovascular Laboratory to determine what symptoms might predict a positive tilt table test. We found that inability to stand in place, fainting, and flushing were most likely to predict a positive TTT (sensitivity of 71% and a negative predictive value of 75%). For a summary of the study, click here.
We studied a Norepinephrine Serotonin Reuptake Inhibitor (NSRI) called reboxetine for Pfizer pharmaceuticals. This drug has shown promise for the treatment of CFS/ME and FM. Enrollment is now closed for the original study, but continuing studies are planned for 2008. For more details see the US Clinical Trials site.
We have completed the study of an antiepileptic drug called lacosamide for the control of pain in persons with FM (PWFs). This study was sponsored by Schwarz Pharmaceuticals.
We have completed the study of a fibromyalgia drug, mirogabalin, for Daiichi Sankyo.